NCO/OH Calculations in Formulator
Please read the document titled “Formulator Acid Base Chemistry” to understand some of the basic concepts behind these calculations
1. Create the physical properties under (920) Physical Property Set-up if they are not already there. You will need 3 properties:
a. Eq. Wt. NCO
b. Eq. Wt. OH
c. A third property to indicate the percent weight “reactive”. You will set this property up with a basis of “% wt resin”. YOU MUST USE THIS BASIS FOR THIS PROPERTY.
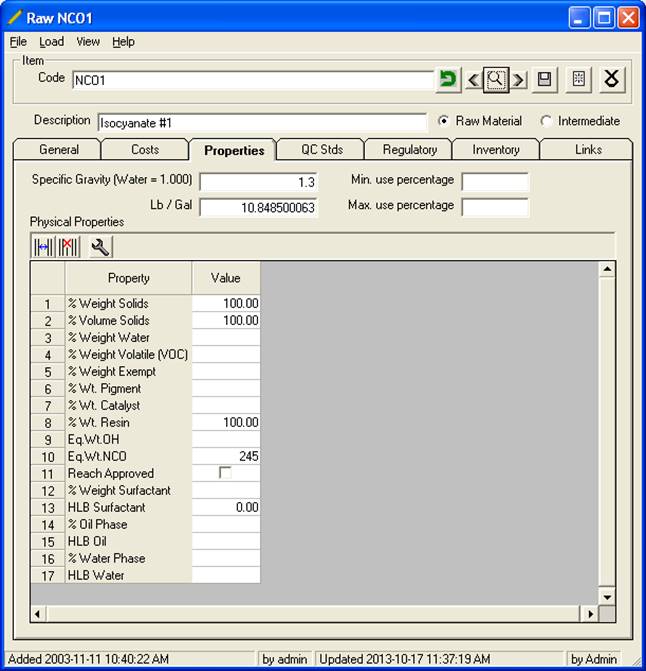
2. Under (10) Raw Material Maintenance enter the values for each raw material. Below is an example of an acid. Note the Eq. Wt. NCO of 245, with a % Wt Resin of 100 (i.e. 100% reactive).

3. Create the equations under (840) Equation Maintenance.
a. When you are creating the equations you will notice 3 columns. You will be using these 3 columns to create your equations.
b. Note: If you scroll down under the first column titled “Quantities” you will notice that there are 4 relative terms at the end:
i. Q_OH is grams of base in the formula
ii. Q_NCO is grams of acid in the formula
iii. [Eq No Base] is the sum of the individual component equivalent numbers
iv. [Eq No Acid] is the sum of the individual component equivalent numbers
v. Equivalent number for an ingredient in a formula will be the ingredient’s weight percent (adjusted for binder content) divided by the equivalent weight of the ingredient.
c. The equation can be built by clicking on the components from the 3 columns or manually typing in the equation. We recommend that you click/select the components from the columns in order to avoid any typographical errors.
d. Here is a sample equation for the NCO/OH index:
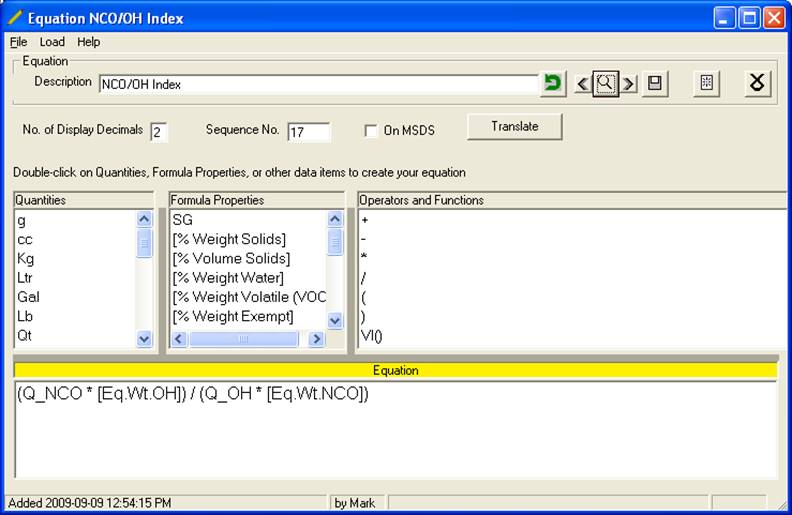
e. Here are the other equations often used:
Desription |
Equation |
Eq.Wt.NCO |
[Eq.Wt.NCO] |
Eq.Wt.OH |
[Eq.Wt.OH] |
Grams NCO |
Q_NCO |
Grams OH |
Q_OH |
NCO/OH Index |
(Q_NCO * [Eq.Wt.OH]) / (Q_OH * [Eq.Wt.NCO]) |
OH/NCO Ratio |
Q_OH / Q_NCO * 100 |